MILAN, Italy – Assistant Professor Christopher H. Hendon shares the theory underpinning an ongoing Coffee Science Foundation research project, supported by Simonelli Group, toward deepening our understanding of espresso extraction. The multi-year research project aimed at measuring espresso chemistry, described below, is an exciting initiative that will correlate those measurements to sensory attributes and lay the groundwork for science-based tools for the industry.
Peter Giuliano, Coffee Science Foundation, explains: “Three years ago, the research team at Simonelli Group reached out at the Coffee Science Foundation, eager to collaborate on cutting-edge espresso research. That initial conversation led to a truly exciting initiative—a multi-year research project aimed at measuring espresso chemistry, correlating those measurements to sensory attributes, and laying the groundwork for science-based useable tools for the coffee industry.”
Giuliano continues: “The reason for this is simple: as technology advances and our understanding of flavor improves, we can put more power in the hands of coffee professionals who seek to make coffee better. It’s this commitment to constant investigation and innovation that created espresso in the first place, and through an ongoing engagement with science and research we can make coffee more enjoyable, more sustainable, and more fruitful for all.
Electrochemistry and espresso
Giuliano adds: “Shortly after Coffee Science Foundation put out a call for research proposals, the well-known coffee scientist Professor Christopher Hendon approached with an idea: could we use the technique known as electrochemistry to measure espresso, to better understand the building blocks of specialty espresso quality?”
There is more: “The idea was enticing and exciting. Dr. Hendon’s proposal led to a Coffee Science Foundation grant, supported by the Simonelli Group, to develop the idea and the technology that might bring it to fruition. For this issue of 25, we asked Prof. Hendon to explain the concept of electrochemistry as it applies to coffee, and how the idea might lead to better tools and techniques for baristas and coffee flavor professionals everywhere. We’re excited to support this work, and committed to sharing it openly with the coffee-science-loving community.”
Using electricity to detect and quantify molecules in brewed coffee
By Christopher H. Hendon
“Coffee can be delicious, but there are many variables that result in highly variable cup quality. Parsing which of these variables makes the largest impact is a challenge.
Is it related to the characteristics of the green coffee, the roasting, the water chemistry, the skill of the barista? Undoubtedly, each plays a key role in producing a tasty beverage, but then there is also the challenge of identifying what’s “tasty”—a subjective characteristic at best—and how it relates to our understanding of “quality,” another complicated word with multiple meanings and interpretations. In coffee, “quality” can indicate the degree of superiority (“quality coffee”), but it can also mean the existence of a property or characteristic (“a coffee’s qualities”). Historically, the coffee industry endeavored to determine absolute measurements of “quality” because such metrics can add value to certain sectors of the supply chain—and then worked to meet established understandings of “quality” across both interpretations.”
The refractive index
“Outside of sophisticated laboratories, one of the ways the industry has historically measured a quality of brewed preparations is by relating coffee’s refractive index to a mass percent concentration (% total dissolved solids, %TDS). The concept is relatively straightforward—the refractive index is simply a measure of effective optical density.
Thus, as more material is dissolved in water, the optical density should increase, and the refractive index should increase—the more coffee you’ve extracted into water, the higher the TDS. And, because we understand how light and water work, we also know that as hot aqueous solutions cool, the optical density and refractive index should also increase. (This is why your TDS levels off and stops increasing once the coffee stabilizes in temperature.)
As you’ve likely experienced, there are a seemingly infinite number of ways to obtain 1.4% TDS as determined from the refractive index—you can even brew the same coffee to the same TDS, and it still may not taste the same, even when using near identical preparation methods! This is because minor fluctuations in optical density, an average of all molecules floating in water, may have a more significant impact on taste.”
The flavor perception
“Our flavor perception may swing dramatically with both major and minor variable adjustments, e.g., brewing two different coffees, or brewing the same coffee at different temperatures. In this case, when we think of TDS as a measurement of quality, we are referring to it in the sense that it provides a statement of a property of the liquid (its concentration), not a degree of superiority of that cup relative to another cup.
As our industry moves into an understanding of specialty coffee based on distinctive attributes rather than a singular notion of “quality,” it would be a significant advance to be able to make statements about both the existence and concentration of particular molecules in a cup of coffee. Further, if we could relate our molecular level insights to a sensory experience, we may then be able to build a more comprehensive understanding (and subjective valuation) of a brewed coffee’s attributes.”
Why electrochemistry?
“In research supported by the Coffee Science Foundation and Simonelli Group, we have begun our pursuit to use electrochemistry to measure the identities and quantities of molecules in brewed coffee. You may be wondering: why electrochemistry? At its core, coffee chemistry is organic chemistry operating within two firm limits: coffee is dissolved in water (a highly polar solvent), and brewed coffee—the solute—is obtained by heterogeneous extraction of organic molecules.
That is to say, when we think of coffee brewing, we think of water contacting solid coffee particles. Since we know that temperature, brew time, brew ratio, pressure, and all the other variables enable access to new flavors within the same bean, electrochemistry offers us a way to “probe” which molecules have been extracted, and how many there are. The idea is to use electricity to determine how families of molecules respond to these standard coffee variables.”
Organic molecules and functional groups
“Without digging too much into the organic chemistry underlying our use of electrochemistry for this purpose, organic molecules contain “functional groups.” The general idea is that molecules with common chemical features should behave similarly when electricity is applied to them.
Although these functional groups do not directly relate to a sensory experience (for example, citric acid tastes very different from quinic acid, even though they share the same functional group), they are relatively easy to identify when we use electricity to briefly move their electrons around, causing the quick oxidation or reduction of these molecules.
These reactions are also often chemically reversible, allowing you to make a measurement of the existence and population of a family of molecules in a solution without destroying them or altering their population in any way. And, perhaps most remarkably of all, these measurements of oxidation and reduction are easy to make—all you need is a device that controls applied voltage and measures current (i.e., how many electrons were passed into the solution).”
Reduction and oxidation
“By adding a couple of inert (non-reactive) metallic electrodes to this device, you can then measure the energy of oxidation and reduction within a solution. Obviously, coffee is extremely complex, with many hundreds of molecules likely featuring similar functional groups. Because each of the functional groups is attached to a slightly different molecule (e.g., methanol is slightly different from ethanol), each molecule will have a specific voltage in which it undergoes reduction/oxidation. As a result, electrochemistry can (in principle) allow us to discern difference in chemical populations of molecules that share common functional groups, too.
Using the device to count the number of electrons passed into or out of the coffee, we have established an approximation that each molecule that responds at a given voltage reacts by one electron, allowing us to conclude that the number of electrons is proportional to the number of molecules in solution. This means that we’re also able to use an electrochemical measurement to deduce the number of molecules in the coffee and make statements about their concentration. Instead of being limited to a cumulative %TDS—an average of all molecules’ impact on the optical density—we can now make direct measurements of the concentrations of families of molecules.
Perhaps more interesting is the fact that this approach can be used to measure reduction and oxidation of molecules with unknown identities. There is no requirement for us to know which particular molecules in coffee are being targeted, but rather to use their existence and quantity to begin to understand how brew parameters—temperature, dose, time, brew method, bean origin/processing method, and other variables—affect these molecular populations.”
The impacts of roast
One example of this can be seen in Figure 1, where we systematically explored the impacts of roast on a single coffee brewed to the same parameters.[1] At around −0.6 V, you can see a clear difference in populations of molecules being reduced, indicating that a family of molecules is destroyed during the roasting process. Now, just think—you could use this feature to identify batch-to-batch variability in coffee roasting.
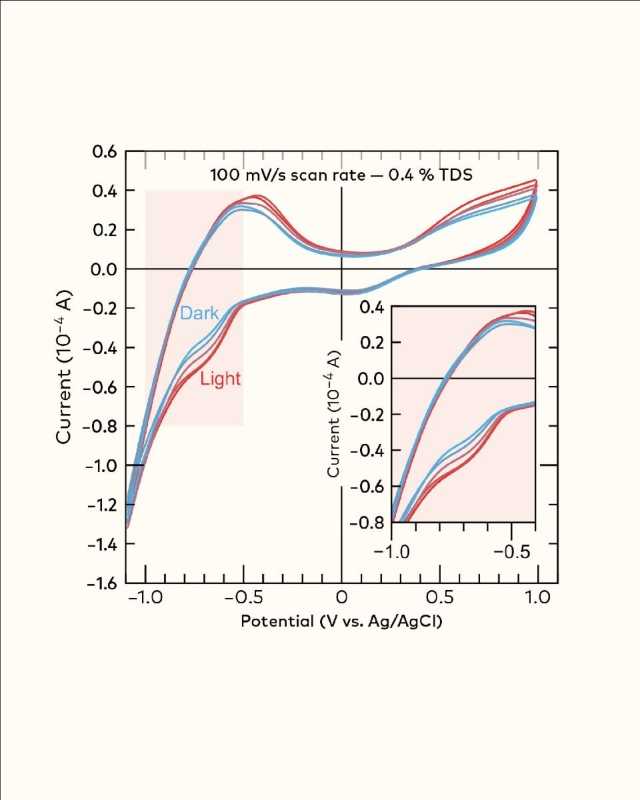
In another example, Figure 2, we brewed two Colombian coffees in two different ways (espresso and filter) and normalized their %TDS; here, you can see clearly that brew method affects the population of these compounds. Now, you might be wondering: what are these compounds? Honestly, we don’t know—yet.
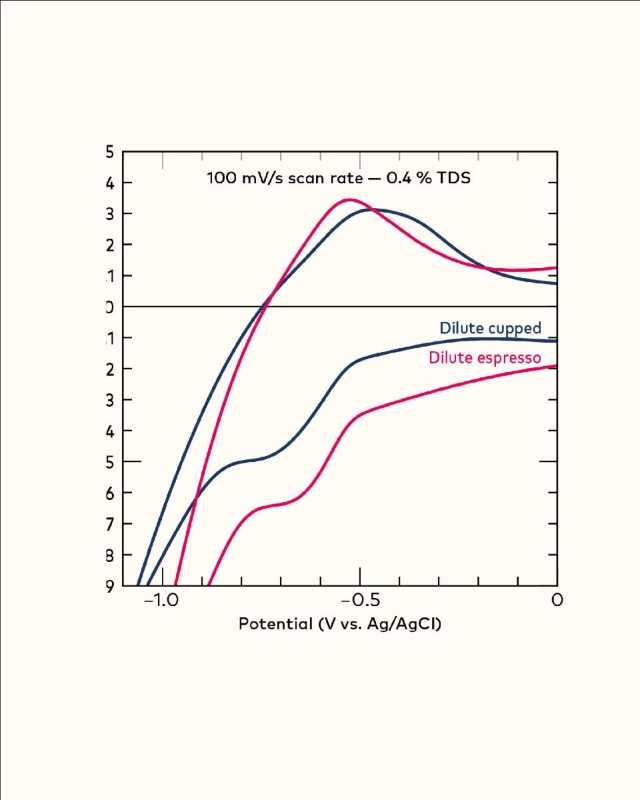
Instead we can speculate on their chemistry based on what we know: brewing under pressure and shorter time results in more of these compounds. We also know they are reducible. This means there is a good chance they are some volatile molecule that is more soluble under pressure, and likely features C=O functionality. Ongoing work at Oregon State University will soon reveal the identity of this family of compounds.ù”
Preparation methods
“Beyond this example, we can imagine several scenarios where these types of measurements become useful. For businesses with many accounts all using the same coffee, an electrochemical measurement will highlight differences between preparation methods.
For roasters batch roasting, the method will allow for quantification of differences in roast. But perhaps the most exciting application of electrochemistry has yet to come—the ability to relate these differences in bulk measurements to exact molecular populations, which we could then relate to a sensory experience. Excitingly, we’re already working in this direction by collaborating with Prof. Elizabeth Tomasino and Prof. Michael Qian, both at Oregon State University, who will assist in the next step of our pursuit to use electricity to measure qualities of coffee.”
About Christopher H. Hendon
Assistant Prof. Christopher H. Hendon is a computational materials chemist at the University of Oregon, where he runs the Hendon Materials Simulation group. Researcher ROBIN BUMBAUGH, a PhD student at the University of Oregon, uses electrochemistry and LC-MS to identify oxidizable compounds present in espresso to use as markers of espresso quality.
This research is ongoing at the University of Oregon and Oregon State University and is supported by the Coffee Science Foundation with underwriting provided by Simonelli Group. Christopher H. Hendon is also supported by the National Science Foundation, the Department of Defense, the Research Corporation, and the Dreyfus Foundation.
The Give and take of electrochemical measurements
“Water, a highly polar solvent, tends to favor the extraction of polar molecules from the coffee, leaving behind those that are less polar (more oily). Although you can affect the relative populations of polar and non-polar compounds by adjusting simple parameters such as temperature and contact time, the extent of extraction (rather than the speed) is governed by two competing energetic factors (the energy released upon solvating a molecule, and the amount of order in surrounding water that molecule necessitates). The reaction, however, is simple:

Since we primarily care about the product—that is, we want to understand more about the coffee extracted into the water—we need a way to probe which molecules have been extracted, and how many there are.
One pillar of organic chemistry is grouping molecules together based on common chemical features. For example, both ethanol (CH3CH2OH) and methanol (CH3OH) feature an –OH group and are therefore both called “alcohols.”
Both formaldehyde (H2CO, a molecule in embalming fluid) and benzaldehyde (C6H5CHO, a molecule that tastes and smells of bitter almonds) share a common HC=O group and are hence “aldehydes.” It turns out that some chemical reactivity can also be linked to the existence of these groups; the arrangement of atoms therefore imparts function and they are hence termed “functional groups.” Unfortunately, functional groups alone do not directly relate to a sensory experience: although citric, malic, and quinic acids are all acids, they all taste remarkably different from one another, despite having common functionality and similar reactivity.”
Furthermore, while the polarity of a functional group does directly affect the optical density of a liquid (which coffee molecules cling to water molecules), the size of the resulting compound molecule dictates its overall impact on optical density.
“For example, both formaldehyde and benzaldehyde have a common functional group, but, per gram, formaldehyde has a much higher optical density in water than benzaldehyde. It’s also important to note that functional groups in organic chemistry are inherently future-proof: all possible connections of atoms to form functional groups have been described, and we use these functional groups as classifiers for families of molecules, independent of whether a molecule featuring them has ever been isolated on Earth.
Some of these compounds will occur in coffee, and their population in a coffee extract may or may not depend on processing method, roast, water chemistry, or barista skill. But one thing is certain—all molecules in coffee have functional groups and we will use their common function to probe their existence. In order to probe functional groups, we can use electrochemistry to selectively perform either of the two following reactions:

We can remember these two reactions from the mnemonic, “An Oil Rig Cat”—Anode, Oxidation is loss, Reduction is gain, Cathode. We can also define electrochemistry to be any chemical reaction where an electron is a reactant or product.
These sorts of reactions are extremely common: oxidation and reduction can then occur between molecules, e.g., the rusting of iron in air to form Fe2O3. In this case solid iron will oxidize (lose electrons) to O2, making a new material—rust.
The electron can also be extracted from (equation 2) or provided to (equation 3) a reaction by applying electricity. In those cases, the energy of the electricity is in the units of Volt, and some reactions will require more voltage than others. For example, it is quite easy to remove an electron from an alcohol, and less easy to extract one from a C–H (alkane) group.
Thus, by applying a particularly low positive voltage we can selectively add and remove electrons from alcohols, but not alkanes. Furthermore, once the electron is removed from the alcohol, the energy to add the electron back in is equal to the energy required to remove it, making the reactions of oxidation and reduction often chemically reversible. This in principle allows you to make a measurement of the existence and population of a family of molecules in a solution, without destroying them or altering their population in any way.”










